How SARS-CoV-2 affects our immune system

After more than three years of pandemic with around 20 million deaths, it is common knowledge that COVID-19 is an insidious disease. The causing virus, SARS-CoV-2, literally kept the world on tenterhooks for a long time. It mainly infects the lung cells and triggers a wide range of symptoms with an often uncertain course of the disease. Thanks to vaccination, the pandemic is largely over, but there are still unanswered questions, such as what the virus actually does in our bodies and how it affects our immune system? Researchers from the Primate Genetics Laboratory and the Infection Models Platform at the German Primate Center have been investigating these questions. Using cultures of human lung cells, they investigated how a SARS-CoV-2 infection affects immune responses in detail. The scientists discovered that a specific peptide in the viral spike protein is responsible for upregulation of a surface protein that is an important factor in the innate immune system. The protein in turn activates adaptive natural killer cells and triggers the new formation of this cell subset in SARS-CoV-2 patients, which could previously only be detected in cytomegalovirus (CMV) infections.
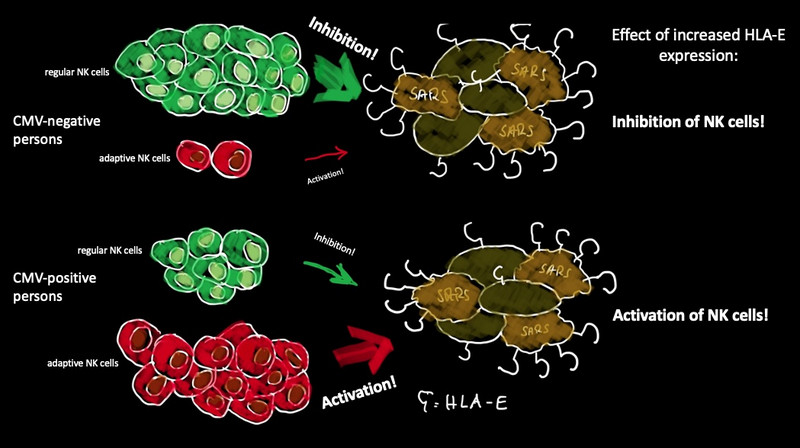
If we become infected with an unknown pathogen, our body is not defenseless against it. Our innate immune system triggers reactions that can effectively kill infected cells. Natural killer cells (NK cells), for example, form a first line of defense in the fight against infections. They can destroy infected cells without having been in contact with the pathogen beforehand. NK cells recognize so-called MHC class I proteins, which are found on the surface of all healthy body cells. These MHC class I proteins also include the HLA-E protein. NK cells can bind HLA-E via both an inhibitory receptor (NKG2A) and a stimulatory receptor (NKG2C) and can be inhibited or activated, respectively. Normally, the NK cells equipped with the inhibitory NKG2A receptor are strongly represented in terms of numbers, while those with the stimulating NKG2C receptor tend to be outnumbered.
However, there are viruses that have changed over the course of evolution in such a way that they can circumvent this first defense strategy of the immune system. A well-known representative is the cytomegalovirus (CMV), which belongs to the group of herpesviruses. The virus manages to ensure that the expression of HLA-E is not down-regulated after infection, as is usually the case, but is actually enhanced on the cell surface. This immune evasion strategy of the virus inhibits the NK cells with the inhibitory NKG2A receptor and sustains viral multiplication.
Another special feature is that CMV infection in humans can often lead to the increased formation of so-called adaptive natural killer cells. This NK cell subset expresses the stimulating NKG2C receptor on their cell surface and can recognize the increased HLA-E on the CMV-infected cells via NKG2C and kill these cells. Adaptive natural killer cells have the advantage over regular NK cells that they react more quickly, are long-lived and have a memory effect. Around a third of all CMV-positive people have a large number of adaptive NK cells. This keeps the latent infection in check, as CMV remain in the body for a lifetime.
The researchers at the DPZ now wanted to find out the influence of SARS-CoV-2 infection on HLA-E expression and the functional consequences on NK cells. To do this, they first infected human lung cells with the virus and observed that the HLA-E protein is induced on the cell surface similar to CMV infection. They obtained the same results after treating the cells only with the spike protein of the virus.
The scientists then carried out a detailed analysis of the spike protein to find out which part might be responsible for the effect on HLA-E. Using in silico analyses, they identified a nine amino acid long peptide as a possible candidate.
“Treating the lung cells with the peptide alone was sufficient to trigger the increased expression of HLA-E,” says Lutz Walter, head of the Primate Genetics Laboratory, summarizing the results. “In addition, we were able to show that the replacement of a single amino acid in the peptide can prevent the increased expression of HLA-E. We were therefore not only able to prove that SARS-CoV-2 induces increased expression of HLA-E, but were also able to show exactly which peptide in the spike protein is responsible for this effect.”
The scientists then analyzed the functional consequences of increased HLA-E expression. To test this, they again treated lung cells with the spike protein. “The CMV status played a decisive role here,” explains Lutz Walter. “In samples obtained from CMV-negative donors, we could see that the function of the NK cells was inhibited after spike protein-mediated increase of HLA-E expression, as these people have much more NK cells expressing the inhibitory NKG2A receptor. In samples from CMV-positive donors, however, we were able to observe a clear stimulation of NK cells through the addition of the spike protein, as these donors contain much more adaptive NK cells expressing the stimulatory NKG2C receptor.” Thus, the immune response of the NK cells to SARS-CoV-2 infection also depends on whether the infected persons are already infected with the cytomegalovirus.
Based on these results, the researchers wanted to find out whether SARS-CoV-2 infection also causes the formation of new adaptive NK cells. To this end, they examined blood samples from hospitalized COVID-19 patients, being either positive or negative for CMV. They were able to show that SARS-CoV-2 infection induced the formation of new adaptive NK cells in all patients regardless of their CMV status. However, a subsequent analysis of blood samples from recovered COVID-19 patients showed that the formation of these new adaptive NK cells was limited to the acute phase of the infection. These additional adaptive NK cells were no longer detectable in recovered patients.
“The fact that the formation of adaptive NK cells can also be caused by a virus other than CMV is a completely new finding,” says Lutz Walter. “The fact that these cells disappear after recovery is probably due to the fact that infection with SARS-CoV-2, unlike CMV, is usually completely cleared. However, this raises the question of whether there is a difference in this respect in Long-COVID patients. It is possible that a virus reservoir remains in the body here, which causes constant stimulation of adaptive NK cells and thus also the different long-term symptoms. Further studies must show whether this is the case.”
The study was funded by the German Research Foundation (DFG) as a joint project by Lutz Walter and Markus Uhrberg (Düsseldorf University Hospital) as part of the COVID-19 focus funding program. In addition to the DPZ and the University Hospital Düsseldorf, researchers from the Fraunhofer Institute for Toxicology and Experimental Medicine (ITEM) and the Hannover Medical School as well as the Leibniz Research Centre for Working Environment and Human Factors (IfADo) at the Technical University of Dortmund were also involved.
Original publication
Hasan MZ, Claus M, Krüger N, Reusing S, Gall E, Bade-Döding C., Braun A, Watzl C, Uhrberg M & Walter, L. (2024): SARS-CoV-2 infection induces adaptive NK cell responses by spike protein-mediated induction of HLA-E expression. Emerging Microbes & Infections. DOI: 10.1080/22221751.2024.2361019